Radioactivity and Nuclear Chemistry
Atomic theory in the nineteenth century presumed that nuclei had fixed compositions. But in 1896, the French scientist Henri Becquerel found that a uranium compound placed near a photographic plate made an image on the plate, even if the compound was wrapped in black cloth. He reasoned that the uranium compound was emitting some kind of radiation that passed through the cloth to expose the photographic plate. Further investigations showed that the radiation was a combination of particles and electromagnetic rays, with its ultimate source being the atomic nucleus. These emanations were ultimately called, collectively, radioactivity.
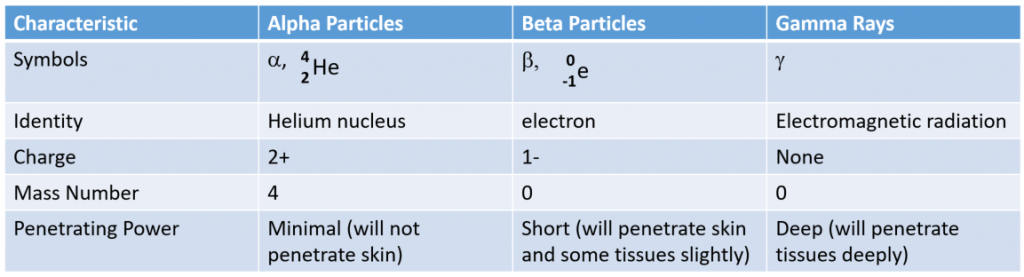
Alpha Particle (α):
Rutherford’s experiments demonstrated that there are three main forms of radioactive emissions. The first is called an alpha particle, which is symbolized by the Greek letter α. An alpha particle is composed of two protons and two neutrons and is the same as a helium nucleus. (We often use 24He to represent an alpha particle.) It has a 2+ charge. When a radioactive atom emits an alpha particle, the original atom’s atomic number decreases by two (because of the loss of two protons), and its mass number decreases by four (because of the loss of four nuclear particles). We can represent the emission of an alpha particle with a chemical equation—for example, the alpha-particle emission of uranium-235.
Beta Particle (β):
The second type of radioactive emission is called a beta particle, which is symbolized by the Greek letter β. A beta particle is an electron ejected from the nucleus (not from the shells of electrons about the nucleus) and has a -1 charge. We can also represent a beta particle as -10e. The net effect of beta particle emission on a nucleus is that a neutron is converted to a proton. The overall mass number stays the same, but because the number of protons increases by one, the atomic number goes up by one. Carbon-14 decays by emitting a beta particle:
Gamma Radiation (γ):
The third major type of radioactive emission is not a particle but rather a very energetic form of electromagnetic radiation called gamma rays.
Nuclear Fission:
Occasionally, an atomic nucleus breaks apart into smaller pieces in a radioactive process called spontaneous fission (or fission). Typically, the daughter isotopes produced by fission are a varied mix of products, rather than a specific isotope as with alpha and beta particle emission. Often, fission produces excess neutrons that will sometimes be captured by other nuclei, possibly inducing additional radioactive events. Uranium-235 undergoes spontaneous fission to a small extent.
CHAPTER SUMMARY
Radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. Six types of radiation produced during nuclear decay were presented within this chapter and include:
- alpha (α) decay which is composed of two protons and two neutrons and has a +2 charge.
- beta (β) decay which is an electron ejected from the nucleus (not from the shells of electrons about the nucleus) and has a -1 charge and no mass. Within the nucleus a neutron emits the electron and is converted into a proton in the process.
- gamma (γ) decay which is characterized by the emission of ionizing radiation and does not contain mass or charge.
- positron (β+) emission which is a positron ejected from the nucleus and has a +1 charge and no mass. Within the nucleus a proton emits the positron and is converted into a neutron in the process.
- electron capture occurs when an inner shell electron combines with a proton and is converted into a neutron. The loss of an inner shell electron leaves a vacancy that will be filled by one of the outer electrons. As the outer electron drops into the vacancy, it will emit energy often in the form of X-rays.
- nuclear fission occurs when an atomic nucleus breaks apart into smaller pieces in a radioactive process that releases excess neutrons.














0 Comments